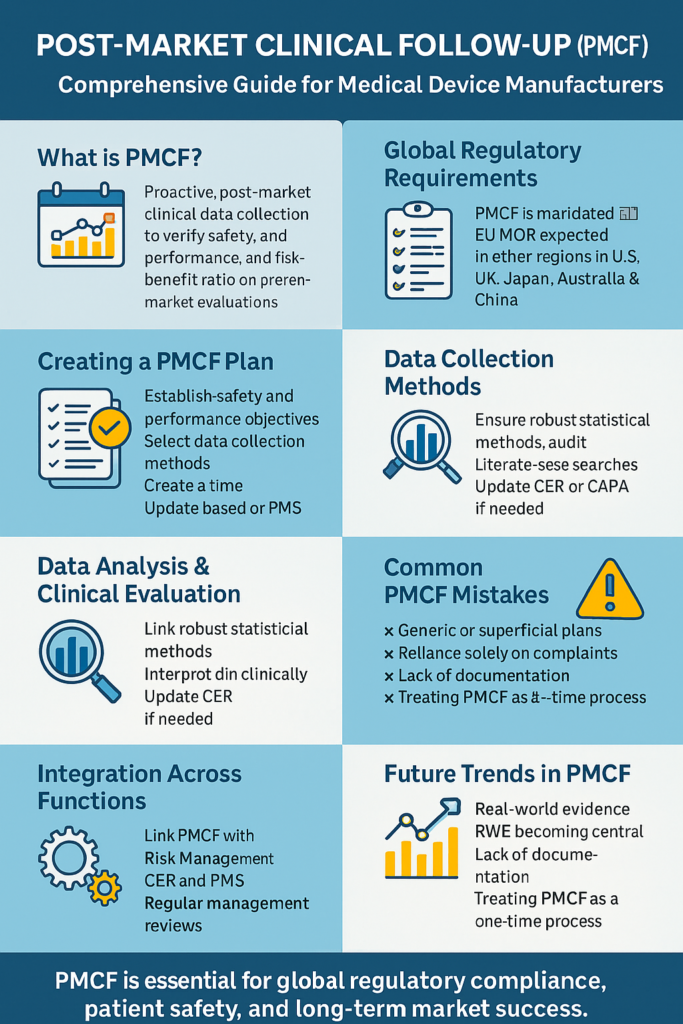
1️⃣ Introduction: Why Post-Market Clinical Follow-Up (PMCF) Is a Global Imperative
It is a high-speed medical device era; market approval is just the beginning.
An ethical, regulatory, and business imperative is diligent device safety and performance after launch.
Enter: Post-Market Clinical Follow-Up (PMCF).
Post-market clinical follow-up is a proactive process that monitors actual in-use medical device performance outside the clinical test setting of pre-market studies.
🔹What Does Post-Market Clinical Follow-Up Set Out to Do?
- Establish the device’s performance and safety within everyday clinical use.
- Identify unknown risks or complications.
- Validate and refresh the benefit-risk balance over time.
- Provide data for regulatory submissions and audits.
- Enable product development and patient safety initiatives.
Global Regulatory Push for Post-Market Clinical Follow-Up (PMCF): Why Now?
Over the past decade, international regulators shifted from single pre-market approvals to lifecycle management of medical devices.
Key drivers include
- Well-publicized failure events (e.g., metal-on-metal hip resurfacing, transvaginal mesh)
- Greater emphasis on patient safety and risk-based regulation
- Extrapolation beyond early clinical trials is necessary.
📊 Global Statistics That Highlight the Post-Market Clinical Follow-Up (PMCF) PMCF Imperative:
EU Notified Body Audits (2023):
Over 35% of audit findings addressed inadequate Post-Market Clinical Follow-Up (PMCF) planning or implementation under EU MDR (Source: EU Notified Body Joint Report, 2023).
FDA Post-Market Studies (2022):
Due to adverse event signals, the FDA requested 57 post-market surveillance studies under Section 522. (Source: FDA 522 Studies Database, 2022).
Global Device Recalls (2021):
Over 70% of high-risk recalls were due to issues that may have been caught beforehand through effective Post-Market Clinical Follow-Up (PMCF) processes. (Global Medical Device Safety Network Report).
🔥 Real-World Regulatory Case Example:
Case—Suspension of CE Mark Due to Ineffective PMCF
In 2022, a manufacturer’s CE mark for a European Class III cardiovascular medical device was put on hold because they did not properly carry out their Post-Market Clinical Follow-Up (PMCF) activities to address known problems that occurred after the product was on the market.
The Notified Body stated:
❌ Inadequate PMCF study plans
❌ Inadequate incorporation of PMCF data into risk assessments
❌ Inadequate CER update
✅ 2️⃣ Regulatory Foundations of Post-Market Clinical Follow-Up (PMCF)—Global Overview
🇪🇺 A. European Union Post-Market Clinical Follow-Up (PMCF) under EU MDR 2017/745
The EU Medical Device Regulation (MDR 2017/745) has structurally changed post-market circumstances for medical devices put on the market in the European Union.
✅ Key EU MDR Post-Market Clinical Follow-Up (PMCF) Requirements:
Elaborated in Annex XIV, Part B, PMCF is an obligatory, forward-looking procedure for any device, regardless of class.
MDR Official Definition of Post-Market Clinical Follow-Up (PMCF):
“A recurring process that explains the clinical evaluation shall form part of the manufacturer’s post-market surveillance plan.”
✅ Key PMDR Post-Market Clinical Follow-Up (PMCF) Deliverables
- Post-Market Clinical Follow-Up (PMCF) Plan (within Post-Market Surveillance Plan)
- Post-Market Clinical Follow-Up (PMCF) Evaluation Report (PMCF ER)
- Post-Market Clinical Follow-Up (PMCF) data integration into the Clinical Evaluation Report (CER)
- Submission of regular PSURs for Class IIa and higher devices
What are the MDR requirements in a post-market clinical follow-up (PMCF) plan?
The Post-Market Clinical Follow-Up (PMCF) Plan should clearly specify the intended use population.
- Clearly defined objectives regarding clinical performance and safety
- Data collection methods (literature, user complaints, studies)
- Statistical approach and procedure for evaluation
- Balance of risk and benefit
- Schedule for review and update
🚫 Regulatory Risks in EU MDR if Post-Market Clinical Follow-Up (PMCF) Non-Compliant:
- CE Mark withdrawal or suspension
- Negative audit findings
- Increased scrutiny from Notified Bodies
- Market access delays
🇺🇸 B. United States—Post-Market Studies Under FDA Oversight
The FDA indirectly does not mention the term “post-market clinical follow-up,” but it demands robust post-market data collection by various means.
✅ 1. Post-Approval Studies (PAS)—For PMA Devices
For PMA-approved devices, the FDA can impose ongoing Post-Approval Studies (PAS) to monitor:
Long-term performance and safety
Rare adverse effects
Regular device performance in multicultural populations
PAS Key Features:
Annual reporting obligations
Possible mandatory labeling adjustment based on results
✅ 2. Section 522 Post-Market Surveillance Studies
PMA and specific 510(k) devices can have 522 surveillance studies requested by the FDA when:
- A serious risk is identified post-market
- The device is intended for implant >1 year
- The device is life-sustaining or life-supporting
📝 FDA Real-World Example:
The FDA asked for a 522 study in 2020 on a transcatheter heart valve when post-market report findings of long-term degeneration of the valve were reported.
The company needed to
- Design a multi-center observational study
- Report findings annually
- Update risk-benefit information in labeling
✅ 3. Unique Device Identifier (UDI) and Real-World Evidence (RWE)
The FDA encourages the use of UDI-linked real-world evidence for post-market monitoring.
- Registry data
- Electronic health records (EHR)
- Claims databases
🇨🇦 C. Canada : Health Canada’s Approach to Post-Market Surveillance
Even though there is no Post-Market Clinical Follow-Up (PMCF) requirement at Health Canada, they do require manufacturers to:
- Monitor adverse events
- Employ vigilance systems
- Provide recall and incident reports.
Canada’s Medical Device Regulations (SOR/98-282) emphasize rigorous, proactive market surveillance according to FDA post-market standards.
🇦🇺 D. Australia: TGA Post-Market Obligations
The Therapeutic Goods Administration (TGA) requires manufacturers to
- Implement a Post-Market Vigilance System
- Report adverse events within statutory timelines
- Cooperate in device recalls and safety alerts
Active post-market follow-up is not listed as a mandatory activity, but it is a significant compliance aspect.
🌏 E. Global Harmonization Efforts—IMDRF’s Role in Post-Market Surveillance
The International Medical Device Regulators Forum (IMDRF) has issued guidance to bring international alignment for post-market surveillance.
Key IMDRF documents:
- IMDRF Post-Market Clinical Follow-Up (PMCF) Guidance (IMDRF/PMCF WG/N47FINAL:2020)
- The life cycle approach to clinical evidence emphasizes
Global regulators increasingly expect manufacturers to:
- Incorporate post-market clinical follow-up (PMCF) planning into the device life cycle.
- Harmonize data collection to support global submissions
⚖️ Post-Market Clinical Follow-Up (PMCF) Expectations Summary by Region:
| Region | Mandatory PMCF | Post-Market Data Integration | Risk of Non-Compliance |
|---|---|---|---|
| EU MDR | ✅ Yes | CER, PMS, PSUR | CE Mark withdrawal |
| FDA | ✅ (PAS/522) | Labeling, PMA Supplements | Warning letters, market action |
| Canada | ❌ (Vigilance required) | Incident reporting | Market surveillance action |
| Australia | ❌ (Post-Market Vigilance) | Adverse event reporting | Regulatory enforcement |
| Global | ⚖️ Harmonized expectations | Lifecycle management | Audit findings, loss of market access |
✅ 3️⃣ Building a Rock-Solid Post-Market Clinical Follow-Up (PMCF) Plan: A Step-by-Step Guide for Manufacturers
🧭Why the Post-Market Clinical Follow-Up (PMCF) Plan Is the Pillar of Compliance
Your Post-Market Clinical Follow-Up (PMCF) Plan is your cornerstone document describing how you will proactively collect clinical information about your device after you have launched it to the market.
In EU MDR Annex XIV, Part B, the plan is
- ✅ Mandatory for all devices (even Class I)
- ✅ Part of your Post-Market Surveillance (PMS) system
- ✅ Extremely closely examined during audits and technical document inspections
A well-written Post-Market Clinical Follow-Up (PMCF) Plan satisfies the regulators and increases product safety, reduces liability, and gains market confidence.
✅ What Should a Post-Market Clinical Follow-Up (PMCF) Plan Cover?—Complete Analysis
1️⃣ Device Description and Intended Use
Start by declaring fairly unequivocally:
- The name, type, and model of the device
- It is an essential medical application.
- The intended patient population
- The innovative features of the device or its novelty
Why is it important?
Here, regulators Read over this section to see if your Post-Market Clinical Follow-Up (PMCF) process is tolerable for the risk and sophistication of your device.
🔹 2️⃣ Summary of Clinical Evaluation and Identified Risks
Write a concise but comprehensive summary of:
- The established clinical benefits of the device
- The identified risks based on the Clinical Evaluation
- The unknowns or gaps to be addressed by Post-Market Clinical Follow-Up (PMCF)
Tip:
Connect this to your Risk Management File (ISO 14971) and Clinical Evaluation Report (CER).
3️⃣ Objectives of Post-Market Clinical Follow-Up (PMCF)
Your objectives should be Specific, Measurable, Achievable, Relevant, and Time-bound (SMART).
Common PMCF Objectives:
- Show continued performance and safety in actual conditions.
- Detect new risks or unexpected adverse reactions.
- Validate clinical benefit in new patient populations.
- Ensure the device’s adequacy regarding instructions for use (IFU).
- Gather data to support changes to clinical claims.
4️⃣ PMCF Methods and Procedures
This is the core of your PMCF plan—tell us exactly how you will collect data.
Typical methods are
- Post-Market Clinical Studies
- Device-Specific Clinical Registries
- Systematic Reviews of Literature
- Surveys and Questionnaires of Users
- Analysis of Vigilance and Complaint Data
For each method, specify:
- Study design or data collection protocol
- Sample size and choice criteria
- Study duration/data collection period
- Methods of data analysis
5️⃣ Data Assessment and Statistical Methods
Indicate:
- How will the data collected be analyzed
- Statistical methods utilized (descriptive stats, trend analysis, significance testing)
- Criteria for data validity and reliability
Why is this important?
Regulators require transparent, reproducible analysis techniques.
6️⃣ Post-Market Activity Integration
Describe how PMCF data will:
- Feed in CER updates.
- Feed into risk management reviews.
- Become integrated into Periodic Safety Update Reports (PSUR)
7️⃣ Reporting Schedule and Review Timelines
Describe:
When will PMCF data be analyzed
How are reports updated (typically annually)
What process will be followed for the internal and external release of PMCF results?
8️⃣ PMCF Evaluation Report (PMCF ER)
Assume you will publish a PMCF Evaluation Report that summarizes
- Study findings
- Data interpretations
- Impact on the benefit-risk profile
- Recommendations for corrective or preventive actions
📝 PMCF Plan Example (Simplified Structure)
| Section | Content Example |
|---|---|
| Device Description | Class IIa diagnostic catheter |
| Risk Summary | Potential for device breakage or infection |
| PMCF Objectives | Confirm performance in routine use, detect rare complications |
| Data Collection Methods | Registry study + user surveys |
| Analysis Methods | Statistical trend analysis |
| Reporting Schedule | Annual review with CER updates |
| PMCF ER | To be submitted to the notified body |
🛡️ PMCF Plan in Real Audits—What Auditors Look For
✅ What Regulators Expect to See:
- Clear, logical PMCF Plan in alignment with risk profile
- Definite, quantifiable objectives
- Carefully chosen, justified data collection methodologies
- Connection with PMS and Risk Management
- Evidence of Implementation and Monitoring
🚫 Common PMCF Plan Audit Findings (from EU Notified Bodies):
- ❌ Objectives that are too generic or too general (e.g., “to monitor use of device”)
- ❌ Review cycle not established or incomplete timeline
- ❌ Complete reliance on literature reviews
- ❌ No plans to incorporate findings into the CER or PSUR
💬 Real Case Example:
During a 2023 MDR audit, a vascular access device company was non-conforming because
- The PMCF Plan lacked clearly defined objectives.
- Data collection relied solely on complaints without active means.
- No link to Clinical Evaluation was seen.
Outcome:
❌ The firm was required to submit a Corrective Action Plan and was behind
In CE Mark.
✅ 4️⃣ PMCF Data Collection Methods: Selecting the Right Method for Your Device
🧭Why Selecting the Right PMCF Data Collection Method is Important
Not all risks—and all devices—need the same strategy for Post-Market Clinical Follow-Up (PMCF).
Your decision should be aligned with
- The risk class of the device
- The type of risks and uncertainties identified
- The target patient population and application intended
- Regulatory requirements within your target markets
✅ Remember: Regulators are getting more cautious about manufacturers relying only on passive data collection, like complaints or literature reviews.
✅ Comprehensive Breakdown of PMCF Data Collection Method
✅ 1️⃣ Manufacturer-Sponsored Post-Market Clinical Research
📌 What Is It?
A formal clinical study was conducted post-market approval to gather real-world evidence under controlled conditions.
📊 Best Used For:
- Class III or implantable devices
- New technology or new indications for devices
- Where pre-market data was limited or equivalence-based
✅ Key Features:
- Conducted following ISO 14155:2020 (Good Clinical Practice)
- Requires ethical approval (IRB/EC) and regulatory notifications
- Established with clearly defined endpoints and hypotheses
✅ Benefits:
- ✅ Provides high-quality, controlled data
- ✅ Allows detailed risk-benefit analysis
- ✅ Generally favored by regulators for high-risk devices
✅ Disadvantage:
- ❌ Time and expensive
- ❌ Requires ethical and regulatory approvals
- ❌ Greater logistical complexity
✅ Real-Life Example:
A Class III neurological implant required a 5-year follow-up PMCF study to assess long-term safety post-CE marking.
Findings supported label modifications for device lifetime and informed follow-on product development.
✅ 2️⃣ Post-Market Clinical Registries
What Is It?
A systematic collection of comparable information on device performance at more than a single clinic or patient population.
✅ Best Used For:
- Devices that are in everyday use in clinical practice (e.g., stents, orthopedic implants)
- Long-term follow-up of device performance
- Development of large databases to study trends
✅ Key Characteristics:
- Industry-sponsored, hospital-based, or national/international
- Data are collected prospectively or retrospectively.
- May include patient-reported outcomes
✅ Benefits:
- ✅ Large-scale real-world evidence
- ✅ Less costly than long-term Monitoring
- ✅ Applicable for tracking trends and low-frequency events
✅ Limitations:
- ❌ Not as strong as clinical trial data
- ❌ Requires governance infrastructure and data protection
- ❌ Possibility of data quality and completeness issues
✅ Real-World Example:
The Swedish Hip Arthroplasty Register is a state-level database tracking joint replacement performance.
It helped identify patterns of early failure in some models of implants, prompting post-market corrective steps.
✅ 3️⃣ User Surveys and Questionnaires
What Is It?
Systematic methods for collecting patient or clinician feedback on device usability, safety, and performance.
✅ Best Used For:
- Low- to medium-risk devices
- Usability testing and real-world use experience
- Capturing subjective performance information
✅ Key Characteristics:
- Must be developed and tested systematically
- Must yield a statistically significant sample
- Answers must be anonymous and objectively analyzed.
✅ Pros:
- ✅ Quick and cheap
- ✅ Provides direct user information
- ✅ Can uncover off-label use or unforeseen problems
✅ Cons:
- ❌ Vulnerable to response bias
- ❌ Limited to self-reported experience
- ❌ Not a substitute for clinical evidence in higher-risk products
✅ Real-World Example:
A surgical stapler manufacturer used structured surveys post-market to identify usability concerns among surgeons, leading to design modifications and new instructions for use.
✅ 4️⃣ Systematic Literature Reviews
What Is It?
A systematic, formal literature search to obtain clinical evidence relevant to the device or similar products.
✅ Best Used For:
- To supplement available clinical data
- Upgrading the Clinical Evaluation Report (CER)
- Gathering new scientific evidence after market launch
✅ Key Features:
- Need to be in line with systematic review principles (e.g., PRISMA guidelines)
- Search and inclusion/exclusion criteria are clearly defined.
- Critical appraisal of data quality is necessary.
✅ Benefits:
- ✅ Cost saving
- ✅ Access to a large pool of clinical evidence
- ✅ Regulators will be pleased with it if done well
✅ Limitations:
- ❌ May lack device-specific real-world data
- ❌ Cannot take over active PMCF for high-risk devices
- ❌ Vulnerable to publication bias
✅ Real-World Example:
A wound dressing manufacturer performed an annual literature review post-market to monitor emerging safety and efficacy data and enabled CER updates for EU MDR.
✅ 5️⃣ Vigilance Data, Complaint Analysis, and Adverse Event Reporting
What Is It?
Reactive data that are collected through post-market surveillance systems, including
- Adverse event reports
- Vigilance databases (e.g., Eudamed Vigilance, FDA MAUDE)
- Customer complaints
✅ Best Used For:
- Enabling active PMCF practices
- Detection of emerging risk signals
- Facilitating trend analysis and risk management
✅ Key Characteristics:
- Mandatory reporting obligations
- Sedately integrated into PMS systems
- Must feed into CER and risk management
✅ Benefits:
- ✅ Real-time safety information
- ✅ Mandatory compliance with global regulators
- ✅ Risks that are potentially identified
✅ Drawbacks:
- ❌ Reactive, not proactive
- ❌ Typically incomplete or delayed
- ❌ Unable to replace planned PMCF activities
✅ Real-World Example:
Assessment of adverse event reports on a diagnostic catheter revealed a manufacturing defect necessitating a voluntary product recall.
📝 How to Choose the Right PMCF Data Collection Method(s)
| Method | Best For | Risk Class | Pros | Cons |
|---|---|---|---|---|
| Clinical Investigation | New tech, implants | III, IIb | High-quality data | Costly, complex |
| Clinical Registry | Long-term monitoring | IIb, III | Broad, real-world data | Data consistency |
| Surveys | Usability insights | I, IIa | Fast, low-cost | Subjective |
| Literature Review | Evidence support | All | Efficient | Limited to published data |
| Vigilance | Safety signals | All | Regulatory requirement | Reactive only |
✅ 5️⃣ Analyzing PMCF Data: How to Transform Raw Information into Actionable Insights and Compliance Evidence
🧭 Why PMCF Data Analysis Matters
Gathering is half the battle of PMCF.
The actual value in product development and satisfying the regulator lies in the interpretation and analysis of the data.
⚡ Regulators expect manufacturers to submit evidence that PMCF data has been statistically analyzed, critically appraised, and directly linked to clinical safety, performance, and benefit-risk profiles.
Incorrect analysis = Non-conformity = Action by the regulator.
✅ The PMCF Data Analysis Workflow Step by Step
✅ 1️⃣ Data Collection Validation and Pre-Processing
Pre-analysis, check that
Data were gathered according to the PMCF Plan
Ethical standards and data protection legislation (e.g., GDPR) have been respected.
Data are not replicated or altered.
Anonymization procedures were maintained.
Tip: Maintain a data integrity log — this is often audited.
✅ 2️⃣ Data Categorize by Type and Source
Organize your PMCF data in functional categories, including:
Adverse events
User experience of device performance
Clinical study outcomes (success rates, complication rates)
Literature outcomes
Example:
If analyzing data on a cardiac device:
Extract adverse event reports from registry follow-up results
Classify complications by severity.
✅ 3️⃣ Use Correct Statistical Methods
Your choice of analysis depends on data type and PMCF purpose.
???? a) Descriptive Statistics
What it does: Provides a summary of raw data (means, medians, ranges, percentages)
Use when: Examining survey responses, event rates, and literature findings.
Example:
5 out of 500 implanted devices led to mechanical failure = 1% failure rate.
???? b) Trend Analysis
What it does: Detects trends or patterns over time
Use when: Reviewing registry data, trend of complaints, trends in adverse events.
Example:
A rising trend of device calibration complaints can be an early indicator of a manufacturing flaw.
???? c) Signal Detection Techniques
What it does: Detects statistically significant trends in adverse events
Use when: Reviewing vigilance data or adverse event reports.
Example:
Comparison of observed rates for events to those of a known baseline rate to look for safety signals.
???? d) Comparative Analysis
What it does: Compares device performance between subgroups or other devices.
Use when PMCF studies or registry analysis are used.
Example:
Comparison of the complication rate between age groups with registry data.
???? e) Root Cause Analysis (RCA)
What it does: Analyzes the underlying cause of events
Use when: There is frequent device malfunction or serious adverse events.
✅ 4️⃣ Clinical Data Interpretation
Regulators require clinical context, not figures.
For each finding:
Judge clinical significance
Refer to known device hazards or new hazards.
Compare this with the results for the intended use population.
Rebalance the benefit-risk profile.
Example Interpretation:
A higher rate of infection post-implantation, however small, will not alter the risk profile if within acceptable clinical limits — but an abrupt increase would require immediate action.
✅ 5️⃣ Recording Findings for Regulatory Submissions
You will need to document clearly:
What data were analyzed
What methods were used
What conclusions were drawn
How findings affect safety, performance, and labelling
???? PMCF Findings to Update with Key Documents:
Clinical Evaluation Report (CER)
Risk Management File
Periodic Safety Update Report (PSUR)
Post-Market Surveillance Report (PMSR)
✅ 6️⃣ Data Insight-Based Action
Analysis needs to lead to:
Design changes (as needed)
Label changes
CAPA (Corrective and Preventive Actions)
Additional training or Instructions for Use
Further PMCF activities should be performed if new risks are detected.
??? Real Audit Finding Example:
Finding:
The manufacturer submitted PMCF data with frequency counts without clinical interpretation or statistical analysis.
Regulatory Finding:
“Data submitted is not interpreted meaningfully enough; benefit-risk reassessment not yet final.”
Impact:
Non-conformity was detected, and the technical file was rejected until the revised one was received.
✅ Best Practices for PMCF Data Analysis
✅ Apply standardized statistical methods
✅ Quantitative and qualitative analysis both included
✅ Clinical interpretation is always presented
✅ Methodology explained in an open form
✅ Involve cross-functional teams—RA/QA, clinical, and biostatistics
✅ 6️⃣ PMCF, Clinical Evaluation, Risk Management, and PMS: Creating an Integrated Compliance Ecosystem
???? Why Integration is Essential to Regulatory Compliance
The most common trap that trap makers get into is taking Post-Market Clinical Follow-Up (PMCF) as a standalone activity — an annual check-box task.
PMCF is Part of an extended feedback loop connecting:
✅ Clinical Evaluation
✅ Post-Market Surveillance (PMS)
✅ Risk Management (ISO 14971)
✅ Corrective and Preventive Actions (CAPA)
???? PMCF is not solely a report.
It is a process of adaptation that supports your entire device lifecycle compliance strategy.
✅ 1️⃣ PMCF and the Clinical Evaluation Report (CER)
???? How They Relate:
The Clinical Evaluation Report (CER) per EU MDR must be updated continuously with post-market data, primarily garnered through PMCF activities.
✅ PMCF Supplements the CER By including:
Providing real-world evidence of device safety and performance
Confirmation or denial of pre-market benefit or risk claims
Confirmation of new risks or benefits not considered initially.
Supply of information for the benefit-risk assessment paragraph
???? Real-World CER Update Example:
A CER for a cardiovascular stent was revised based on PMCF evidence of the restenosis rates projected below, the improved benefit-risk profile, and the justification for extended indications.
✅ 2️⃣ PMCF and Post-Market Surveillance (PMS)
???? How They Relate
PMCF is a new component of the Post-Market Surveillance System, as outlined in EU MDR Article 83.
✅ PMCF Feeds Into PMS By:
Supplying systematically collected data regardless of complaint management
Spotting trends in device performance or adverse events
Enabling periodic review by PMS Reports or PSUR
???? Example of Synergy of PMS & PMCF:
One firm found increasing complaint rates for kinking of catheters. PMS launched a PMCF survey that detected the issue under specific conditions of use, and a design modification was implemented.
✅ 3️⃣ PMCF and Risk Management (ISO 14971)
???? How They Relate
ISO 14971:2019 emphasizes that risk management is a lifecycle process, not just pre-market work.
✅ PMCF Enables Risk Management By:
Providing new knowledge about existing or newly recognized risks
Verifying that risk controls continue to perform as designed in real environments
Identifying newly identified risks
Triggering risk analysis revision and control action
???? PMCF Example of Impact on Risk Files
A firm applied PMCF outcomes to identify an unusual failure mode of a battery, revising the risk management file and implementing a design change.
4️⃣ PMCF and Corrective and Preventive Actions (CAPA)
???? How They Relate
PMCF results generally trigger Corrective and Preventive Actions (CAPA) by discovering risks, non-conformities, or unexpected performance trends.
PMCF Triggers CAPA By:
Implies deviation from expected performance
Identifying systemic problems that need to be corrected
Triggering preventive measures to minimize new risks arising
???? PMCF Triggering CAPA Example:
PMCF registry results indicated increasing infection rates with a novel surgical implant. CAPA was triggered, resulting in surgical technique training and a revised IFU.
✅ 5️⃣ PMCF’s Role in Technical Documentation and Audits
???? Why It is Important:
The regulators of today expect manufacturers to demonstrate how PMCF data are proactively addressed in:
The Technical Documentation (Annex II and III of EU MDR)
Risk Management Reports
PMS and PSUR Reports
Clinical Evaluation Documentation
???? Most Common Audit Results Associated with PMCF Integration:
❌ Findings of PMCF without association with Risk Management activity
❌ CER updates that fail to include post-market data
❌ PSUR reports that do not include PMCF-led conclusions
❌ CAPA reports without mention of PMCF data
✅ 6️⃣ Best Practices for Smooth PMCF Integration
✅ Maintain PMS, PMCF, and Risk Management data in a centralized database
✅ Establish cross-functional review groups for PMCF outputs
✅ Integrate Quality Management Systems (QMS) with built-in automatic alerts for future risks
✅ Educate RA/QA personnel about the place of PMCF within the broader compliance schema
???? Next Section Coming Up
✅ 7️⃣ PMCF in Practice — Real-Life Case Studies of Global Medical Device Manufacturers
The case studies shall demonstrate how PMCF works, with success stories and regulatory fiascos.
Next in the following message!
You said:
Next
ChatGPT said:
Thanks!
Let us proceed to Section 7 — PMCF in Practice: Real-Life Case Studies of Global Medical Device Manufacturers.
✅ 7️⃣ Post-Market Clinical Follow-Up (PMCF) in Practice — Real-Life Case Studies and Lessons Learnt
???? Why Real-World Experience Matters
Learning about PMCF from regulatory guidance is vital, but observing how manufacturers have applied PMCF in practice successfully (and sometimes not so successfully) can be truly enlightening.
These case studies are drawn from reported regulatory Enforcement, published reports, and industry audit findings.
✅ Case Study 1: Orthopedic Implant PMCF Avoids Market Recall
???? Background:
An EU MDR European manufacturer brought a cementless hip prosthesis to market.
Literature reviews and equivalence data were utilized in the pre-market clinical evaluation.
✅ PMCF Plan Implemented:
A clinical registry was launched in five hospitals with a manufacturer’s sponsorship.
Complication rates and patient-reported outcomes (PROs) were followed up for 3 years.
Adverse events and revisions were followed up half-yearly.
???? PMCF Outcomes
Early results showed a higher revision rate due to implant loosening among patients below 55.
Root cause analysis revealed this was due to dissimilar bone density in younger patients.
???? Corrective Actions:
Amended IFU with age-specific requirements
Enhanced surgeon training on implant choice
Informed the Notified Body of PMCF results and corrective actions
✅ Outcome
The CE mark persisted without a fine
Excellent audit comment on proactive PMCF
Better market reputation as a responsible manufacturer
✔ Case Study 2: Failure of PMCF and Enforcement by Regulatory Agencies of Device Manufacturers
???? Background:
The manufacturer of a Class IIa rapid diagnosis device failed to have active PMCF activities post-marketing the product.
They had no active data collection or user surveys.
They merely relied on passive complaints monitoring and vigilance reports.
???? Audit Results:
PMCF Plan: generic and non-specific
Active data collection or user surveys were not performed.
No updates in Clinical Evaluation Reports in terms of new scientific evidence
❌ Action Regulatory Taken:
The Notified Body reported a Major Non-Conformity
90 days were provided for the manufacturer to rectify the PMCF deficiencies
Marketing of the device was suspended pending demonstration of compliance.
???? Lessons Learned:
❗ PMCF must be proactive and device-related
❗ Literature review and complaint tracking are NOT adequate for PMCF
❗ Delays in PMCF can jeopardize market access
✅ Case Study 3: FDA Mandated Post-Approval Study Following Adverse Events
???? Background:
An FDA-cleared Class III heart valve had unexpected post-market thrombosis-related adverse events.
✅ FDA’s Post-Market Action:
Issued a 522 Post-Market Surveillance Order
Featured provision for a prospective, multi-center observational study of thrombosis rates
Annual reporting with risk-benefit reassessment
???? Manufacturer’s Response:
Created a compliant PAS with endpoints negotiated with the FDA
Rolling PAS findings into the labeling and risk management system
Expanded Monitoring in foreign markets based on US findings
✅ Outcome:
Prevented product recall
Protected regulator confidence
Improve product safety profile through risk-informed redesign.
✅ Case Study 4: Successful Literature-Based PMCF with Regular CER Update
???? Background:
A wound care medical device company utilized a systematic literature review for its PMCF process.
✅ How They Succeeded:
Used PRISMA-informed literature review approach
Conducted quarterly reviews to monitor new evidence
Updated CER with each significant finding
Integrated literature with regular surveys to healthcare professionals
✅ Result:
Encouraging feedback from Notified Body auditors
No significant non-conformities for three successive audits
Vision of transparency and clinical watchfulness
✅ Common Themes For These Cases
Positive Practices Risky Practices
Ongoing data collection: Serial complaint dependency
Regular CER updates Passive PMS without PMCF
Incorporation with risk management. Foggy PMCF plans
Regulator communication Oblivion of the PMCF plan implementation
✅ 8️⃣ Common Mistakes in Post-Market Clinical Follow-Up (PMCF) and How to Avoid Them
???? Why is it crucial to be aware of common PMCF mistakes
Regulators, especially under the EU MDR, are giving more importance to Post-Market Clinical Follow-Up activities.
Most companies do not have non-conformities because they ignore PMCF, but because they do it superficially or inaccurately.
⚠️ Superficial or erroneous PMCF = Regulatory risk, Suspension of CE mark, or FDA questioning.
✅ Most Common PMCF Mistakes Encountered in Actual Regulatory Audits
❌ 1️⃣ Adhering to PMCF as a One-Time Process
The Mistake:
Manufacturers write a PMCF Plan once, collect scant data, and think they have done it.
Why It is a Problem:
PMCF has to be a continuous process, with continuous review and reconsideration balanced against clinical Monitoring, PMS, and risk management.
How to Avoid:
✅ Make PMCF a rolling exercise with recurring review periods
✅ Rebaseline the plan based on trends in real-world data
✅ Align PMCF activities with your risk review process
❌ 2️⃣ Exclusive use of Passive PMS Data (Complaints, Vigilance)
The Error:
Misconception that complaints and vigilance data are adequate for PMCF.
Why It is a Problem:
PMCF requires active data gathering, not reactive investigation of readily available reports.
How to Steer Clear:
✅ Make active use of tools like surveys, registries, or clinical studies
✅ Use passive data as a supplement to active follow-up
✅ Document your approach in the PMCF Plan
❌ 3️⃣ Generic PMCF Plans and Reports
The Error:
Copy-and-pasting templates or generic objectives in PMCF Plans.
Why It is a Problem:
Regulatory agencies require device-specific PMCF with definite, quantifiable objectives regarding already determined risks.
How to Avoid:
✅ Make all PMCF Plans device-class and risk-profile-specific
✅ Establish SMART objectives (Specific, Measurable, Achievable, Relevant, Time-bound)
✅ Link PMCF deliverables to device safety and performance statements
❌ 4️⃣ Omitting Literature Reviews or Conducting Them Inadequately
The Error:
Omitting literature reviews or conducting them through outdated or non-specific methods.
Why It is a Problem:
Literature reviews are acceptable for capturing changing clinical evidence, provided they are done systematically.
How to Avoid:
✅ Conduct with PRISMA or similar structured review protocols
✅ Document search strategies, databases utilized, inclusion/exclusion criteria
✅ Use peer-reviewed material and rule out outdated publications
❌ 5️⃣ Failure to Combine PMCF with CER, PMS, and Risk Management
The Mistake:
Isolating PMCF results and not updating documents like the Clinical Evaluation Report or Risk Management Files.
Why It is a Problem:
Regulators expect convergence — PMCF results must affect your clinical claims, risk evaluations, and PMS activities.
How to Avoid:
✅ Reference PMCF results against CER updates
✅ Update risk files according to PMCF results
✅ Include PMCF results in your PMS Plan and PSUR
❌ 6️⃣ Not Properly Documenting PMCF Activities
The Mistake
Executing PMCF but failing to document procedures, findings, or follow-up measures.
Why It is a Problem:
Regulators will only consider it as not done if it is not documented.
Poor documentation = audit non-conformity.
How to Avoid Getting in Trouble:
✅ Keep proper PMCF logs, analysis reports, and correspondence
✅ Use controlled templates and versioned documents
✅ Produce clear PMCF Evaluation Reports for each cycle
❌ 7️⃣ Delays in PMCF until After Audit Findings
The Mistake:
Waiting for a regulator to identify PMCF vulnerabilities before acting.
Why It is a Problem:
Reactive PMCF undermines compliance credibility and can result in requests for correction actions or penalties.
How to Avoid:
✅ Perform PMCF actively in your PMS Plan
✅ Schedule routine review and internal audit
✅ Use CAPA to close PMCF gaps on a timely basis
✅ Bonus: Increasing PMCF Mistakes Due to Digital Tools
Overreliance on computerized analysis without verification by human personnel
Reliance on AI-generated literature reviews without verification
Taking electronic registries as compliance with the regulation without Monitoring
✅ PMCF Mistakes to Avoid Often
✅ Customize your PMCF plan to each device and location
✅ Pro-act — do not anticipate waiting for something to go wrong
✅ Write everything down to regulatory-level precision
✅ Educate all relevant departments in PMCF roles and actions
✅ Embed PMCF into your Quality Management System (QMS)
✅ 9️⃣ The Future of Post-Market Clinical Follow-Up (PMCF): Trends Worldwide, Real-World Evidence, and Digital Transformation
???? Why Manufacturers Need to Stay Abreast of PMCF Trends
Regulatory science never rests.
PMCF requirements are changing in response to:
Emerging medical technology innovations at breakneck speed
Accelerating digital health solution advancements
Increased global regulatory harmonization
Increased scrutiny of real-world data (RWD) and real-world evidence (RWE)
???? Staying abreast of these trends can mean the difference between seamless compliance and costly regulatory hold-ups.
✅ 1️⃣ Real-World Evidence (RWE) at the Center of PMCF
???? What is real-world evidence (RWE)?
Data from real-world sources such as
Electronic Health Records (EHR)
Insurance claims databases
Patient registries
Wearable device data
Mobile health applications
✅ Why RWE matters for PMCF:
Gives insight into the performance of the device in larger, unselected populations
Detects rare adverse events before controlled trials
Offers long-term outcome data at an affordable price
???? Real-World Example:
The FDA RWE Program has informed label expansions and regulatory decisions with RWE from large registries and EHRs.
In 2021, RWE enabled clearance of an expanded indication of a cardiac monitor without extra clinical trials.
✅ 2️⃣ Expansion of Regulatory Acceptance of Digital Solutions in PMCF
???? Digital Platforms for Data Collection in PMCF:
Cloud-based clinical registries
AI-based data analysis tools
Blockchain for data traceability and integrity
Wearable sensors for continuous Monitoring
✅ Opportunities with Digital Solutions:
✅ Automated collation of data across multiple sources
✅ Monitoring real-time risk dashboards
✅ Increased traceability and audit readiness
✅ Integration of PMS and QMS systems
❗ Warning:
Software tools are no replacement for the regulatory process they must remain compliant with data protection (e.g., GDPR), validation, and audit trail obligations.
✅ 3️⃣ The Rise of Patient-Reported Outcomes (PROs) in PMCF
???? Why PROs Are Becoming More Relevant:
Individual patient feedback on their experience with the device
Captures usability, satisfaction, and clinically not always reported adverse events
Coalesces with regulators’ interest in patient-centered data
✅ Regulatory Encouragement:
The EU MDR officially legitimizes the role of patient input in clinical evidence generation.
The FDA’s Patient-Focused Drug Development Initiative encourages using PROs in clinical assessment.
✅ 4️⃣ Global Harmonization of PMCF Requirements
???? The Role of the IMDRF (International Medical Device Regulators Forum):
Working together to create harmonized post-market clinical follow-up guidance
Makes recognition of PMCF activities easier across jurisdictional boundaries
Reaches out to eradicate duplication in global submissions
✅ Example Harmonization Efforts
IMDRF N47: Post-Market Clinical Follow-Up Guidance Document
Joint industry-regulator initiatives on post-market surveillance
✅ 5️⃣ The Growing Role of Artificial Intelligence (AI) in PMCF
???? Applications of AI in PMCF:
Predictive analytics for signal detection
Natural Language Processing (NLP) to review literature and complaint data
Automated adverse event pattern recognition
✅ AI’s Advantages in PMCF:
✅ Processes high volumes of big data quickly
✅ Picks up faint risk alerts early
✅ Enables perpetual watch models
❗ Regulatory Caveat:
Regulators anticipate AI systems to be auditable and transparent, and validated traceability of AI-informed decisions is anticipated.
✅ 6️⃣ Getting Ready for the Future What Manufacturers Ought to Do Now
✅ Invest in End-to-End Post-Market Data Systems
Individual systems for PMCF, PMS, CER, and Risk Management
Cloud-based systems with regulatory-grade security
✅ Set up a Multidisciplinary PMCF Team
Clinical, Regulatory Affairs, Quality, Data Science, IT
Regular cross-functional review of PMCF deliverables
✅ Keep Current with International Regulatory Directives
Subscribe to IMDRF news
Monitor FDA, EU MDR, Health Canada, and TGA guidelines.
Industry conferences and working groups
✅ Train Teams on Upcoming PMCF Expectations
Regular workshops on real-world evidence standards
Digital compliance tool training and data governance training
Continuous learning culture in RA/QA teams
✅ ???? Conclusion: Post-Market Clinical Follow-Up (PMCF)—Your Compliance Lifeline and Competitive Advantage
???? Why PMCF Is No Longer Optional It is essential.
The global regulatory landscape has changed to regulating medical devices throughout their lifecycle.
Post-Market Clinical Follow-Up (PMCF) is leading the way both as a safeguard against the risk of non-compliance and as an incentive for product development.
✅ PMCF is not a burden but a critical business asset to maintaining market access, patient safety, and stakeholder confidence.
✅ Summary of What You Have Learned in This Comprehensive Guide
✅ PMCF Is a Continuous, Proactive Process
PMCF must be ongoing, with structured data collection that validates your device’s safety and performance in real-world settings.
✅ Global Regulators Demand PMCF Integration
EU MDR to the FDA, Health Canada, and beyond, PMCF is the global expectation; non-compliance can be catastrophic.
✅ Your PMCF Plan Is the Key to Success
A well-written PMCF plan:
Sets precise objectives
Defines good data collection practices
Describes statistical analysis protocol
Seamlessly integrates with CER, PMS, and risk management.
✅ Data Analysis Makes PMCF Actionable Intelligence
Statistically sound analysis:
Provide for clinical evaluation updates
Facilitates risk-benefit reassessment
Launches product improvements and corrective actions
✅ Integration Across Functions Is Not Optional
PMCF must drive:
Clinical Evaluation Reports (CER)
Post-Market Surveillance (PMS)
Risk Management Activities
Corrective and Preventive Actions (CAPA)
✅ Real-World Evidence and Digitalization of PMCF
Effective use of RWE, patient-reported outcomes, and AI tools can enhance PMCF performance, provided they comply with regulations.
✅ Worldwide Harmonization Calls for the Future of PMCF
The IMDRF and the goliath regulatory bodies are coming together; manufacturers must prepare for multi-jurisdictional PMCF submissions.
✅ Last Word: PMCF Is an Opportunity, Not an Obsession
Manufacturers who view PMCF as a competitive advantage will build stronger market positions.
Build stronger market positions
Improve patient outcomes
Reduce regulatory risks
Create a culture of continuous improvement.
✅ PMCF is your gateway to being compliant, competitive, and in the global realm of medical devices.
???? Quick Summary — 10 Key Points:
1️⃣ EU MDR and future global regulations require PMCF
2️⃣ . It has to be ongoing, proactive, and documented
3️⃣ . Your PMCF Plan guides your strategy and compliance
4️⃣ . Choose data collection methods by risk and type of device
5️⃣ Logically analyze data and provide clinical interpretation
6️⃣ Feed PMCF results into your CER, PMS, and risk management
7️⃣ . Avoid common pitfalls like weak documentation or open-ended plans
8️⃣ Use RWE, digital solutions, and AI responsibly and effectively
9️⃣ Stay up to date with global regulatory directions
???? Apply PMCF in your Quality Management System as a standard procedure
📝 References
- EU MDR 2017/745 — Official Journal
- FDA Post-Approval Studies Database
- ISO 14155:2020 Clinical Investigations Standard
- IMDRF Post-Market Clinical Follow-Up Guidance
- FDA Real-World Evidence Program Framework
You May Also Like
Labeling Success Blueprint: How to Achieve FDA and EU MDR Compliance with Confidence
EU Clinical Evaluation with FDA Clinical Data Strategies: Empowering MedTech Success Globally
