The classification of medical devices under FDA and EU MDR is made simple. Learn how accurate classification drives compliance, approvals, and market success.
🧭 Why Understanding Medical Device Classification Is Critical
- What type of submission you will be required to make (510(k), PMA, De Novo, or CE Mark)
- Whether or not a Notified Body or FDA premarket approval will be required on your product
- How much clinical, technical, and post-market information is required
- Which quality processes and risk management are used
⚠️ Incorrect classification is one of the most common (and expensive) mistakes made by MedTech startups and manufacturers. It can lead to:
- Delayed product launch
- Rejected submissions
- Recalls or audit results
🔍 What Is Medical Device Classification?
EU and FDA medical device classification is a risk-based system that groups devices into classes based on their intended use, invasiveness, and criticality. Both regulatory systems. FDA in the United States and MDR in the European Union—use multi-class frameworks to assess safety and regulatory requirements.
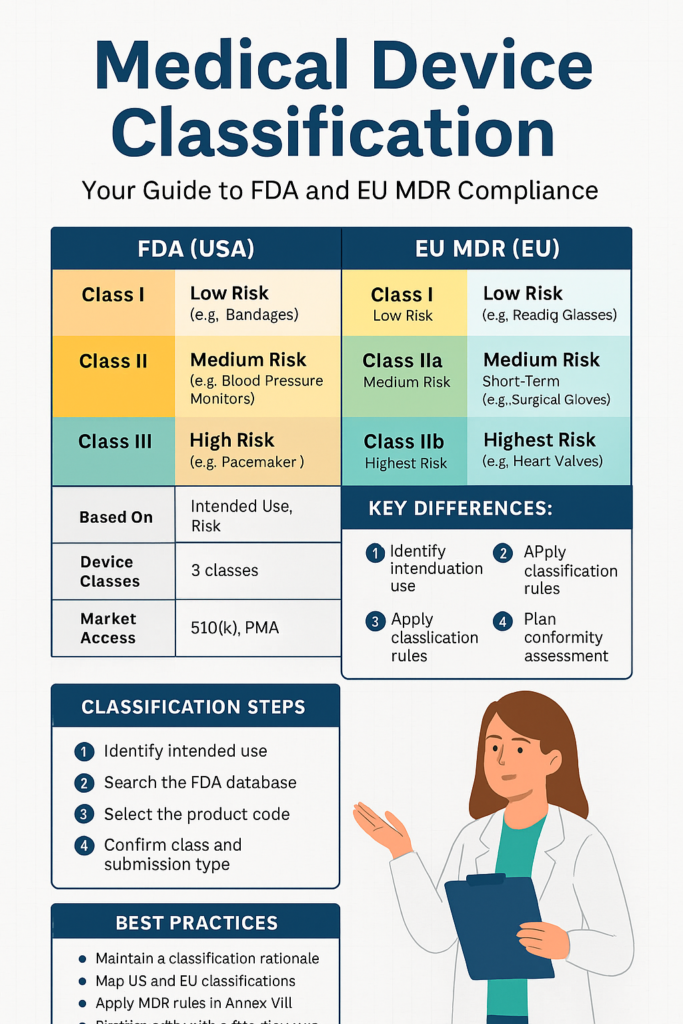
| Region | Classifications | Based On |
|---|---|---|
| FDA (USA) | Class I, II, III | Intended use + risk |
| EU MDR | Class I, IIa, IIb, III | Duration, invasiveness, anatomical site, etc. |
US FDA Classification: Explained in Detail
FDA Classification: Simplified in Detail
The United States Food & Drug Administration (FDA) has classified medical devices into three classes:
✅ Class I: Low-Risk Devices
Examples:
Bandages
Manual surgical instruments
Thermometers
Key features:
Subject to General Controls (e.g., labeling, registration, complaint resolution)
Most exempted from premarket notification (510(k))
Self-certified using eSTAR or FURLS of FDA
Why it matters:
These are easy to market but require a quality management system good under 21 CFR Part 820.
✅ Class II: Moderate-Risk Devices
Examples:
Blood pressure meters
Syringe pumps
Contact lenses
Important features:
Subject to General + Special Controls
Generally require a 510(k) submission
Must be shown to be substantially equivalent to a legally marketed predicate
Why it is essential:
Most new, networked, and computerized healthcare devices fall into this Class. Proficient predicate selection and documentation are critical.
Read: FDA 510(k) Submission Checklist
✅ Class III: Devices of High Risk
Examples:
Implantable defibrillators
Pacemakers
Deep brain stimulators
Key Features
Life-sustaining, life-supporting, or hazardous devices
Require a Premarket Approval (PMA)
Require clinical trial data, biocompatibility, and complete manufacturing verification.
Why it is important
This is the most time-consuming and expensive route. Most startups make the mistake of submitting inappropriately for PMA when a De Novo or 510(k) would be sufficient.
FDA Tips for Success:
Use the FDA Product Classification Database to search for:
Product code
Regulation number
Class and submission type
Search for analogous devices and their regulatory history.
Submit to the FDA via Q-submission to request a classification explanation.
EU MDR Classification: Deep Dive
The EU MDR 2017/745 uses four basic classes and 22 classification rules defined in Annex VIII.
✅ Class I: Low-risk devices
Examples:
Reading glasses
Crutches
Non-invasive monitoring cuffs
Key features:
No Notified Body (NB) involvement for the majority of the devices
Must prepare Technical Documentation and affix a declaration of conformity.
For Class Is (Sterile), Im (Measuring), and Ir (Reusable Surgical), NB involvement is required.
Why it matters
It is the shortest path to CE marking, but Annex I GSPRs, UDI, and PMS compliance have not been waived.
✅ Class IIa: Short-Term, Medium-Risk
Examples:
Dental materials
Infusion tubing
Short-term catheters
Key features
Notified Body validation of Technical Documentation must be performed
PMS, PSUR, and PMCF (Post-Market Clinical Follow-up) planning needs to be performed
Why it matters
Most wearable sensors, medical app devices, and diagnostics come under Rule 11 here.
✅ Class IIb: Increased Risk, Long-Term
Examples:
Surgical lasers
Long-term ventilators
Infusion pumps
Key Features:
Active surveillance and risk-benefit justification are necessary
PMCF and trend reporting are necessary
✅ Class III: Highest Risk
Examples:
Heart valves
Drug-eluting stents
Devices constructed using biological materials
Key Features:
Required clinical studies and NB inspection
Full conformity assessment paths (Annex IX, X, XI)
Notified Body routine audits and EUDAMED registration
🧩 How to Determine Classification Step-by-Step
🔹 For FDA
1. Specify intended use and technological features
2. Search products in the FDA Classification Database
3. Match your device to a predicate and verify:
- Class I (exempt)
- Class II (510(k))
- Class III (PMA/De Novo)
4. Review guidance documents and relevant standards.
5. If unsure Submit a 513(g) or Q-submission.
🔹 For EU MDR:
1. Identify device duration (transient, short-term, long-term)
2. Identify invasiveness (external, invasive, surgically invasive)
3. Check Annex VIII classification rules (Rule 11 specifically for software)
4. Apply the highest relevant rule.
5. Map to conformity assessment procedure (e.g., Annex IX)
6. Confirm with the Notified Body, if required.
Read: Step-by-Step EU MDR Submission Guide
⚠️ Common Mistakes to Avoid
❌ Using MDD classification logic for MDR
❌ Classifying software as Class I
❌ Omitting Rule 11 for AI or computer products
❌ Assuming “general wellness” claims are synonymous with FDA exemption
❌ Not documenting your reason for classification in your Technical File
💡 Best Practices
✅ Keep a Classification Justification Document
✅ Do dual-classification mapping for FDA + EU
✔ Begin classification activities before the design freeze
✔ Document your reasoning in your Risk Management File
✔ Establish NB alignment early (particularly for new devices)
